Antibiotics
Antibiotics are substances that kill or inhibit the growth of bacteria. Antibiotics occur naturally in the environment and are produced by bacteria and fungi where they are thought to be used in defence against other bacteria or to send chemical messages as means of communication. Because of their ability to kill or stop the growth of bacteria, we use them as medicines to treat bacterial infections in humans and animals.
Antibiotic resistance
Antibiotic resistance means the bacteria is resistant, not the human or animal that is being treated. There are many different families of antibiotics, which are used to treat different bacterial infections. Some antibiotics are specific for certain bacteria whereas others are active against a range of bacteria. They are known as broad-spectrum antibiotics. Some bacteria can become resistant to more than one antibiotic – multiply resistant – and they are often referred to as superbugs. Antibiotics are not effective against other types of microbe such as viruses or fungi. For that you need anti-virals or anti-fungal reagents.
When antibiotic resistance occurs, antibiotics do not work and some infections can no longer be treated. Resistant bacteria can also persist and be spread to other people or animals. Bacterial resistance to antibiotics is a very important global issue; it is reported that resistant bacteria will cause 10 million deaths by 2050. Routine and complicated surgical operations would be unable to be performed due to the risk of infection and mild infections that we do not worry about today could become deadly in the future.
Discovery of antibiotics
Although antibiotics have been used for millennia in traditional medicine, it was only in the last century that people began to understand what antibiotics were and that they could be used to treat bacterial infections. Everything started with Sir Alexander Fleming, a Scottish scientist who accidentally discovered penicillin. In 1928, he noticed that a fungus, Penicillium notatum, had contaminated a petri dish containing a Staphylococcus bacterial culture, which he had casually left uncovered before a two-week holiday. On his return, he observed a zone of inhibited bacterial growth surrounding the mould (a bacteria-free zone).
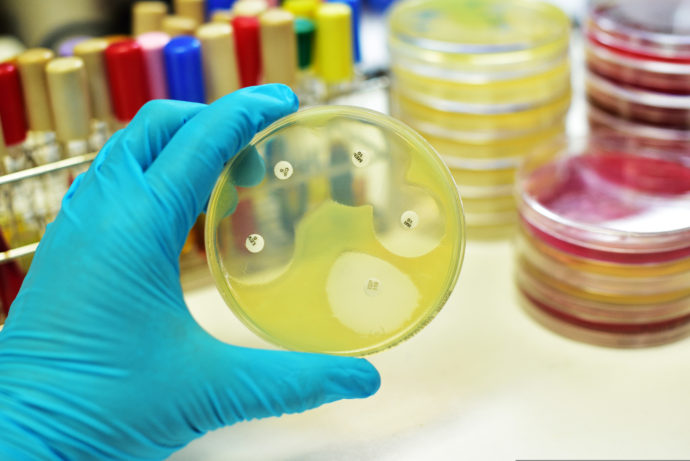

However, it took over a decade before penicillin was introduced as a treatment for bacterial infections in medicine. In fact, the first commercially available antibiotic was a sulphonamide, with the brand name “Prontosil” developed by Gerhard Domagk who won the Nobel Prize in Physiology or Medicine in 1939. Penicillin was very difficult to produce and purify in large doses and in 1941, driven by the number of soldiers requiring treatment with infected wounds in World War II, a major effort began to improve production. This included growing the Penicillium notatum mould in large volumes and purifying penicillin in sufficient quantities.
By 1944, penicillin was being mass-produced and saved thousands of lives by the end of the war. In 1945 a Nobel Prize was awarded to Sir Alexander Fleming jointly with Ernst Boris Chain and Sir Howard Walter Florey for their work on penicillin. From there, antibiotics caused a revolution in healthcare, but resistance has developed in many bacteria that cause serious diseases. This has occurred primarily because of their overuse or because they have been used incorrectly.
Development of resistance
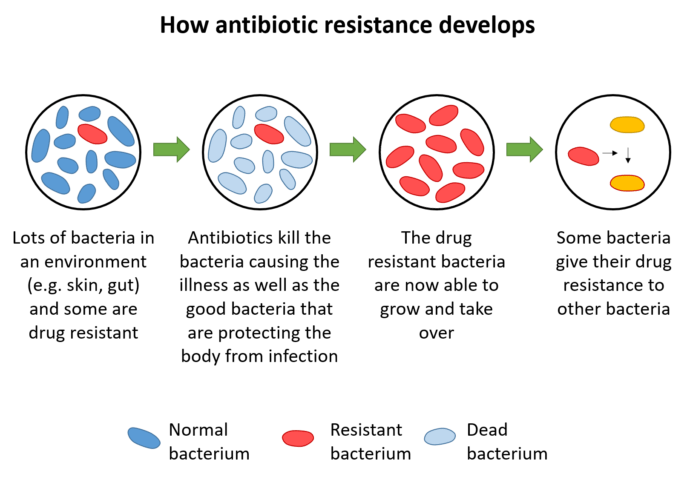
Antibiotic resistance occurs naturally in nature but it can develop and spread more rapidly when bacteria are exposed to antibiotics. Because antibiotics have been overused and used inappropriately in healthcare and farming since their discovery, this process has been greatly accelerated and a large number of bacteria are now resistant. Bacteria can evolve, through changes in their DNA or acquisition of new pieces of DNA and these changes allow them to resist the effects of the drugs. These resistant bacteria can then also be spread due to poor hygiene and inefficient infection control. An example of antibiotic misuse is taking an antibiotic to treat the common cold or the flu, which are caused by viruses and not bacteria.

Where does this happen?
Resistance often develops in hospitals. Infections in hospitals can occur more frequently because people with health problems may not be able to fight off infections as well as a healthy person and require antibiotics as a result. Bacteria can also spread very easily between patients, hospital staff and visitors. Because of this, good hygiene practices such as hand washing are vital. This simple action is still the best way to fight against nosocomial (hospital acquired) infections, especially for hospital staff.
Antibiotics were also heavily used in agriculture for disease treatment of animals, and in sub-therapeutic levels to prevent infections and as growth promotors to make animals grow faster. There are now laws to prevent overuse from happening in the UK and EU and although the use of antibiotics by farmers has reduced greatly, this is not regulated in all countries. Resistant bacteria that emerge on farms can then be transferred to humans directly, via food or via the environment.

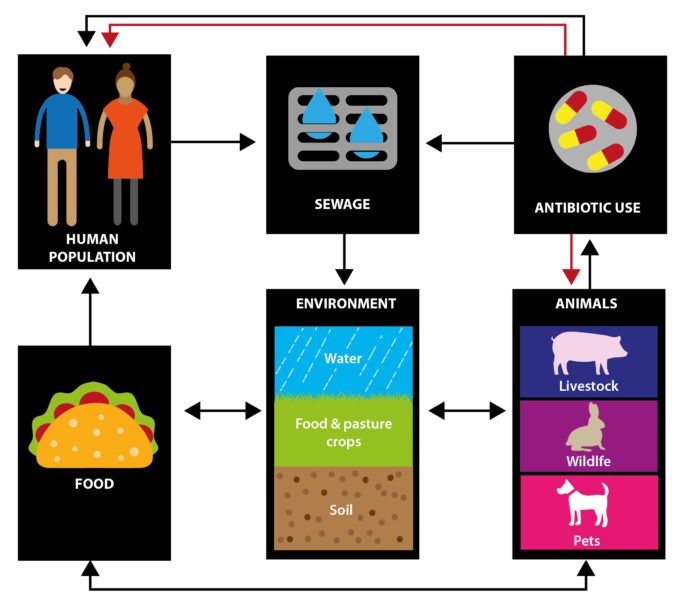
Once resistant bacteria have evolved, they can also be spread. This includes transmission within the community, from person to person or from animals to people and vice versa. Bacteria can also be spread through the environment, and scientists are trying to understand how resistant bacteria are spread between farms, sewage, hospitals effluent, wildlife and water bodies. These bacteria can also be spread globally through travel, food importation, and migratory wildlife populations. Over recent years, people have become more aware of the connections between humans, animals and the environment and this is known as One Health.
Additionally, because some antibiotics are very stable and may persist in water systems, their presence could be a driver of resistance in the environment. Scientists predict that antibiotic resistance genes may be spread more readily in increased temperatures, so global warming could also increase the problem.
How do we stop antibiotic resistance?
There are certain things that we can all do to limit the development of antibiotic resistance. You should complete courses of antibiotics even if you feel better, only use prescribed antibiotics and never share antibiotics or use leftover antibiotics. Good hygiene is important to limit the spread of resistant bacteria and avoid infections, and you should keep vaccinations up-to-date.
In medicine and hospitals, good hygiene is vital. Professionals should test to understand what is causing an infection and to confirm antibiotics are needed and prescribe antibiotics at the right dose, for the correct duration.
In the agricultural sector, antibiotics should only be given to animals to control or treat infections and these should be prescribed by a vet. Farming practices must also follow good hygiene and biosecurity practices and animals should be not be allowed to get stressed, which can increase the development of infections.


Scientists also have important and wide-ranging roles. Some are working towards improved vaccines and diagnostics for humans and animals. This is to reduce the requirement for antibiotics, and ensure that infection is diagnosed quickly and correctly so the appropriate treatment is given. Other scientists are trying to discover new antibiotics so we do not run out of treatments. Scientists also help to improve our understanding of how bacteria and their genes are spread in the environment.
Finally, governments and policy makers also have an important role in developing policies and supporting strategies to reduce the use of antibiotics and raise awareness of this important issue.
Read also
Gut health
Antibiotics and your gut
Antibiotics are used to treat bacterial infections but they can also ‘kill’ our gut microbiota and this can impact on our health.
